What is Reverse Osmosis and How Is It Used For Industrial Applications?
Reverse osmosis has been around as a water treatment process since the late 1950s, and it continues to be an effective process for many separation applications today. If you’ve heard of this technology before, but you want more clarity on what reverse osmosis is and how it works, you’ve come to the right place. Read on to learn more about the science of reverse osmosis, and how it works for a variety of industrial water treatment, separation, and wastewater treatment applications.
What is reverse osmosis?
Before we get into reverse osmosis (RO), it’s helpful to start with a basic explanation of osmosis. By definition, osmosis is the natural tendency of solvent molecules to move in order to equalize solute concentration on either side of a semipermeable membrane. If that sounds like a mouthful, think about it this way: imagine a container filled with water (or another solvent). Say you were to insert a semipermeable membrane to divide the container in two. The semipermeable membrane contains pores that are only large enough to admit solvent molecules—in this case, water. So while the water is able to flow through the membrane, things like solute ions, molecules, or particulates that are present in the water are generally too large to fit easily through the pores.
Next, say you were to add salt (or another solute) to one side of the container. The water molecules would travel through the membrane from the no-salt side, and accumulate on the side where salt was added, effectively diluting the solution on the salt side. This spontaneous movement of solvent molecules from an area of low solute concentration to an area of high solute concentration is the process known as osmosis. Water will continue to flow through the membrane by osmosis in order to equalize the solution concentration on either side of the semipermeable membrane.
As a solvent flows through the membrane, you may end up with a higher volume of liquid on the receiving side. The difference between the two sides is known as osmotic pressure, which is defined as the amount of pressure needed to stop the net flow of pure solvent to an area of high solute concentration. In nature, osmosis is a vital process that allows plant and animal cells to exchange water, nutrients, and minerals through their cell walls and cell membranes.
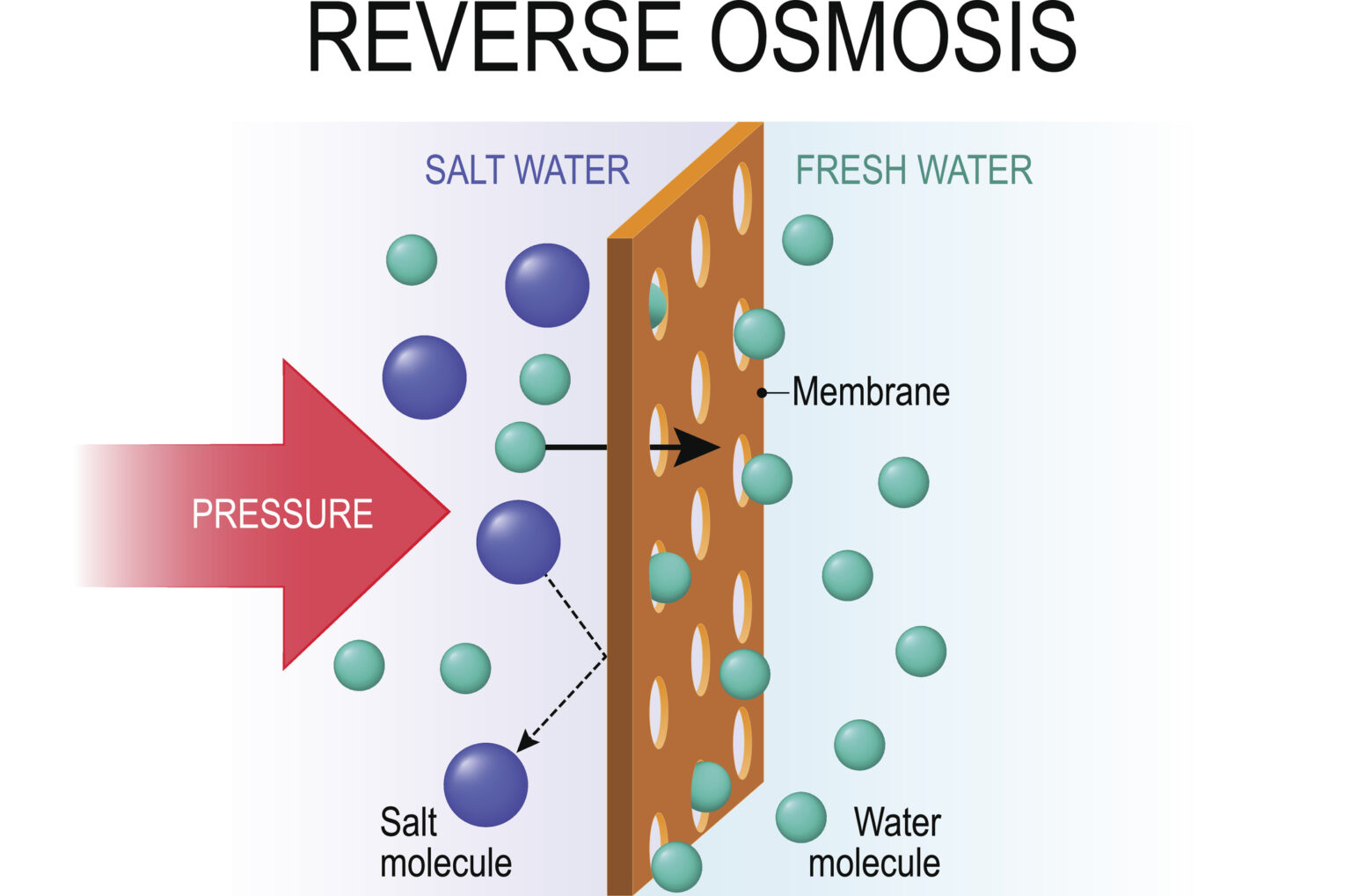
Reverse osmosis (RO) turns this on its head. By definition, reverse osmosis is a pressure-driven separation process used to purify water by passing it through a semipermeable membrane, thereby separating it from other constituents. Essentially, the solvent flows in the opposite direction from natural osmosis, hence the term “reverse” osmosis.
How do reverse osmosis systems work?
Reverse osmosis works by applying pressure to a liquid stream to direct its flow through a semipermeable membrane that filters out dissolved solids and particles from the water (or other solvent). RO systems employ cross-flow filtration, meaning that the liquid flows over the surface of the membrane, and as it does, the pure solvent molecules permeate through the filter.The RO membrane prevents most solutes or other contaminants from passing through, and they flow out of the system as a highly concentrated reject stream. Meanwhile, any water that has crossed through the membrane exits the system as a purified permeate stream.
As we’ve said, RO systems work against the natural process of osmosis. To do so, they require pressure to be applied to the feed stream. The amount of pressure applied must be enough to overcome the osmotic pressure of the solution, and will vary depending upon variables like solute concentration and temperature.
What contaminants does reverse osmosis remove?
RO has the smallest membrane pore sizes of any membrane separation technologies, and is therefore capable of more complete separation than microfiltation, ultrafiltration and nanofiltration. RO removes contaminants based on particle size and charge, and is capable of separating out ions and molecules down to 0.0001 µm, such as:
-
-
- metals and minerals (e.g. calcium, fluoride, potassium, lead, iron, manganese, chromium)
- dissolved salts
- sulfates, detergents, and surfactants
- Nitrates and nitriles (e.g. cyanide)
- total dissolved solids (TDS)
- some taste-, color-, and odor-producing compounds
- some organic contaminants and pesticides
- some pyrogens and pathogens (e.g. bacteria and viruses)
-
While RO is great for removing a broad range of contaminants, its effectiveness can be somewhat limited for removal of dissolved gases, volatile organic contaminants, and some types of pesticides, solvents, and pathogens.
How is reverse osmosis used in industry?
Over the next few years, analysts are anticipating accelerated growth in the RO membrane market, indicative of wider use of RO across many industries. The driving factors behind this growth includes rapid industrial expansion, as well as increasing attention to water scarcity issues. Indeed, RO can offer some advantages, including energy efficiency, a smaller footprint, and appropriateness for water recycling and reuse systems. For these reasons, RO is used in a variety of applications across many industries, including the following common applications:
-
-
- Power: RO has been used within the power industry for decades. Its applications here include boiler feedwater treatment and other pretreatment applications in order to prevent scaling and other problems in thermal systems, promote safe operation, prolong equipment service life, and increase cooling tower cycles. Additionally, RO technology is increasingly being employed for resource recovery from power industry wastewater, with reclamation efforts targeting water, energy, minerals, and other resources.
- Refinery: Beyond its main application for producing high-quality purified water, RO is also useful for concentrating materials found in feedwater. Refinery facilities that want to recover valuable resources from their process and wastewater streams can do so by collecting the RO reject stream. Examples of such applications include a Canadian refinery’s use of RO for lithium recovery, as well as a Kansas refinery’s use of both microfiltration and RO to reclaim wastewater from a nearby municipality.
- Petrochemical & chemical: Since chemical industries require high quality water to ensure consistency and safety on their production lines, they often turn to RO for process and raw water treatment. But its usefulness doesn’t end there, as chemical and petrochemical facilities also use RO for wastewater treatment applications in order to seize on benefits such as reduced wastewater volumes and discharge costs.
- Oil & gas: The oil and gas industry uses RO for produced water treatment, wastewater treatment, and water recovery, as well as desalination of seawater in order to produce potable water aboard offshore drilling rigs.
- Mining & metals: RO is effective for removal of metals, suspended solids, and sulfates, making it useful for treating mining wastewater and acid mine drainage water. Because RO produces high-quality water, mines and metals facilities sometimes adopt RO for non-potable reuse applications as well.
- Food & beverage: The ability of RO to produce high-quality water makes it suitable for a number of applications within the food and beverage industry, where consistency, quality, and safety are top priorities. Common applications include treatment of ingredient water, process liquids, and boiler makeup water, as well as reduction of biochemical oxygen demand (BOD) in wastewater. RO is also useful for concentrating solvents and process solutions, such as brine, for reuse.
- Municipal: RO is often used for drinking water applications, with systems ranging in scale from those serving a single residence, institution, or community, to those serving entire municipalities. RO is also useful for seawater desalination in areas with limited access to freshwater, as well as more specialized applications, such as treatment of hard well water.
- Pulp & paper: Over the last couple of decades, water and resource conservation efforts have driven increased adoption of RO within the pulp and paper industry. RO can be used for diverse applications, such as treating raw water, replacing conventional wastewater treatment technologies, or anything in between. Especially when paired with other membrane technologies, ion exchange systems, or other pretreatment steps, RO can be a great solution for paper and pulp facilities looking to reduce wastewater volumes, cut energy consumption, and it can also be useful for treating groundwater or process water as well.
- Other industries and applications: RO is also commonly used for rinse water treatment, as it is capable of producing ultrapure, low hardness water that is a useful for applications where water spots are not acceptable, such as in car washes and semiconductor manufacturing.
-
Can SAMCO help?
SAMCO has over 40 years’ experience custom-designing and manufacturing reverse osmosis (RO) systems for a range of industries and solutions, so please feel free to reach out to us with your questions.
For more information or to get in touch, contact us here to set up a consultation with an engineer or request a quote. We can walk you through the steps for developing the proper solution and realistic cost for your RO treatment system needs.
You can also check out our blog to learn more about other industrial filtration and process separation technologies. Some articles that might be of specific interest to you include:
-
- Reverse Osmosis vs Nanofiltration Membrane Process: What Is the Difference?
- What are Microfiltration and Ultrafiltration and How Do They Work?
- Ion Exchange vs. Reverse Osmosis: Choosing the Best Treatment System for Your Needs
- Reverse Osmosis and Nanofiltration Membrane Filtration Systems: Common Problems and How to Fix Them
